Twisting molecule probes cell membrane tension
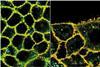
Changing fluorescence visualises strain in live cells, and reveals unusual behaviour
A molecule that can insert into cell membranes and change its fluorescence according to their tension has been developed by researchers in Switzerland. The research has already led to new insights into membrane dynamics.
Cells actively regulate the tension of their membranes as it is crucial to numerous process such as ingestion of particles and division. But measuring this tension in living cells can be difficult or impossible, so gaps remain in biologists’ understanding.