Universal method bypasses obstacles to making neglected hydrocarbon skeletons
Universal method bypasses obstacles to making neglected hydrocarbon skeletons
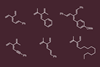
Chemists have developed the first general approach for synthesising dendralenes. The method involves two or three simple steps from commercially available aldehydes and provides access to a wider range of the branched oligoalekenes than previous approaches. It’s also the first method to prepare dendralenes in a stereoselective manner.
