Unprecedented biosynthetic transformation found to connect antibacterial polyketides from ant microbiome
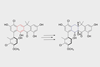
Study identifies enzymes that convert flat fasamycins into three-dimensional formicamycins
Researchers in the UK have identified a unique reaction pathway between two natural products made by the Streptomyces formicae KY5 bacteria that live on ants in Kenya. These compounds, a fasamycin and a formicamycin, have previously been shown to possess potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Understanding how the bacteria turn the flat fasamycins into three-dimensional formicamycins could ultimately help chemists develop new drug leads