Unprecedented natural products uncovered in springtails
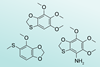
Highly hetero-substituted benzenes serve as chemical deterrents for species of insect-like creatures
Researchers have identified a series of highly hetero-substituted benzenes within mushroom springtails, a species of soil-dwelling six-legged arthropods. The compounds appear to protect the miniscule creatures from ants and one of them represents the first fully hetero-substituted benzene to ever be found in nature.
