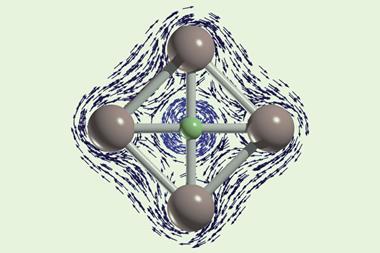
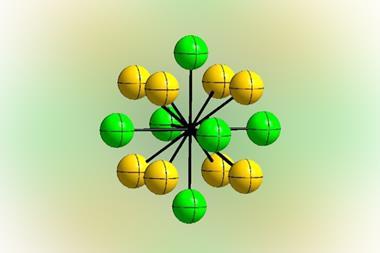
For the first time, chemists have synthesised an interhalogen compound exhibiting a unique central fluorine atom coordinated by four BrF5 groups.1 The one-of-a-kind interhalogen ion in [NMe4][Br4F21]·BrF5 represents the first example of a central tetracoordinated fluorine bridging to neither metal nor hydrogen atoms.
Tetracoordinated fluorine atoms are known in ionic compounds, for example in CaF2. And µ4-fluorine atoms are present in the tetrahydrogen pentafluoride ion and a handful of metal compounds.
Last year, a team led by Florian Kraus from the Philipps University of Marburg in Germany, synthesised [Br3F16]–, 2 which has a triple-coordinated central fluoride ion. Now, building on their previous work, Kraus and co-workers have used a sterically demanding [NMe4]+ counter ion to form the bulkier µ4-fluorine containing interhalogen ion, [Br4F21] –.
![3D rendering of molecule [Br4F21]](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/web/h/m/c/br4f21inarticle_663912.gif)
Halogen fluorides are known for their extreme reactivity and the synthesis did not come without its challenges. The team first tried reacting pure BrF5 with [NMe4]F but a violent reaction between the two compounds caused a small explosion and a day’s work was lost in an instant. On searching for an alternative method they came across research by Karl Christe from 19893 that helped them to reliably access [NMe4][BrF6] and they went on to attempt a controlled reaction of [NMe4][BrF6] with excess BrF5 at low temperatures. It worked. Graham Saunders, a synthetic fluorine chemist from the University of Waikato in New Zealand, comments ‘technically it’s very difficult work and it’s very elegant’.
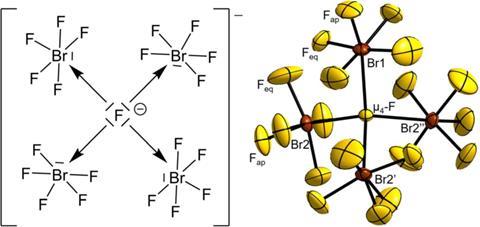
Deciphering the compound’s structure also presented a challenge as it initially appeared very complex. However, by focusing on the centres of the ions and BrF5 molecules, the team discovered its crystal structure was similar to the MgAgAs-type structure. ‘Although the two compounds could not be more chemically different, they are structurally closely related,’ says Marburg team member Martin Möbs. Additional quantum calculations reveal that the µ4-fluorine–bromine bonds are best described as ionic.
‘To encapsulate a fluoride ion in a non-metallic inorganic framework such that it’s bound to four different moieties is really quite extraordinary, because normally you would not anticipate that kind of bonding arrangement,’ comments Thomas Lectka, an organofluorine chemist from Johns Hopkins University, US. ‘It’s an interesting result and I think it will spur more work into the halogens with fluorine,’ adds Saunders.
But while the µ3-fluorine and µ4-fluorine polynuclear anions of BrF5 have now been isolated, Möbs concludes that ‘the anion in which the fluoride ion is surrounded by two BrF5 molecules, [Br2F11]–, is still missing.’
Correction: The images were updated on 12 February 2024
References
1 M Möbs et al, Chem. Sci., 2024, DOI: 10.1039/d3sc06688f
2 M Möbs et al, Chem.–Euro. J., 2023, 29, e202301876 (DOI: 10.1002/chem.202301876)
3 W W Wilson and K O Christe, Inorg. Chem., 1989, 28, 4172 (DOI: 10.1021/ic00321a027)











No comments yet