Iron-rich nanoparticles can catalyse conversion of CO2 to complex organic molecules
Before life on Earth, there had to be reactive organic molecules. Iron-rich nanoparticles from meteorites and volcanism could have catalysed conversion of CO2 on early Earth into such molecules, according to researchers in Germany.
The group mimicked deposits on early Earth using particles from crushed meteorites and volcanic ash. The experiments also replicated the hot conditions of the time (150–300°C), at higher pressures of 10–50 bar and under a wet or dry climate.
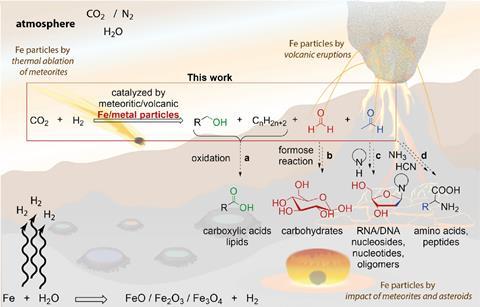
The particles catalysed CO2 conversion in the presence of naturally occurring minerals and atmospheric H2 or H2O in the lab, generating aldehydes, alcohols and hydrocarbons under a wide range of conditions.
‘We saw the formation of lots of these oxygenated reaction products,’ says Oliver Trapp, a chemist at Ludwig-Maximilians University Munich, who led the research. ‘We got a lot of formaldehyde, acetaldehyde and also methanol and ethanol, as well as long chain and branched alkanes.’
Forming oxygenated compounds is exciting, because formaldehyde and acetaldehyde are important building blocks for carbohydrates, amino acids and nucleic acids. The team estimates that 6×108 kg/year of prebiotic organics could have been made from atmospheric CO2 during the Hadean aeon – around 4.5 billion years ago when huge quantities of CO2 were likely released into the atmosphere, along with hydrogen and water vapour.
The experiments indicate that hydrocarbon formation was favoured at higher temperatures, but oxygen-containing organic compounds would have been generated more efficiently as temperatures fell towards 150°C.
‘Previously, there was no explanation as to how large quantities of CO2 in the early atmosphere could have been converted into versatile organic compounds,’ says Trapp. There was a large delivery of meteorites and reduced metals to early Earth, generating hydrogen, he adds, and catalysis in the atmosphere and on the surface converted the CO2 into organic compounds quite rapidly, within perhaps 20–100 million years. ‘Gigatonnes of material were produced. Which means that all the CO2 outgassing from the surface of early Earth was essentially converted into organics,’ says Trapp. ‘This is beyond almost any industrial process we can think of.’
Previously, there was no explanation as to how large quantities of CO2 in the early atmosphere could have been converted into versatile compounds
Astrobiologist Jessica Weber at Nasa’s Jet Propulsion Laboratory in Pasadena, US, who was not involved in the research, draws attention to the complexity of chemistry with and without life. ‘The biotic and abiotic world are very different. Life has taken over on Earth and any abiotic materials now generated would get eaten quickly by life,’ she says. ‘It is not surprising that we don’t see chemistry like this now.’
Jim Cleaves, a researcher at the Earth-Life Science Institute in Tokyo, Japan, says we now have ‘an embarrassment of riches in the ways you can make organic compounds. This is another one to consider.’ But a major mystery remains: ‘A big problem is: okay, you’ve got organics, but how do you put them together to make a living thing. The community is still stuck on that,’ he adds.
Trapp thinks he has an answer to the initial step: activation of aldehydes could have led to the formation of catalysts that essentially became the first photosynthetic systems, he suggests. This is based on his lab’s previous investigations with imidazolidine-4-thione organocatalysts. The most active and selective can create their own building blocks, powered by sunlight, creating a kind of Darwinian evolution on a molecular level.
References
1. S Peters et al, Sci. Rep., 2023, 13, 6843 (DOI: 10.1038/s41598-023-33741-8)
















No comments yet