Tiny, light-powered devices could enable wireless high-throughput electrosynthesis on the microlitre scale. The researchers who designed them hope that the inexpensive devices will reduce the barrier for the broad use of electrochemistry in organic chemistry.
First introduced as a tool for chemical synthesis in the early 19th century, electrochemistry is experiencing a resurgence of interest here thanks to factors that include the desire to avoid toxic, hazardous and expensive reagents. However, the intricate reaction set up has limited its widespread use in the broader organic chemistry community particularly when it comes to high-throughput experimentation, which has become crucial to rapidly develop reactions, particularly in the pharmaceutical industry.
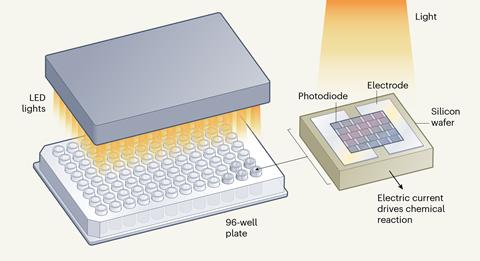
‘You need to wire all the electrodes to a power source,’ explains Song Lin, of Cornell University in New York in the US, one of the researchers on the study. ‘That’s okay if you run five reactions, but if you run 384 reactions, which is the standard for HTE [high-throughput electrosynthesis] in industry, then you need 768 electrodes and wires to connect to power sources and that’s just not practical.’
In a collaborative effort between Lin’s organic chemistry group and Paul McEuen’s physics lab, both at Cornell, the researchers set out to develop a device comprising everything required for electrochemistry that had a convenient energy source and was easy to scale for high-throughput experiments.
Inspired by solar cells, the team developed microelectronic devices called small photoelectronics for electrochemical synthesis (Specs), which are powered only by visible light and convert any traditional 96-well or 384-well plate into an electrochemical reactor. The devices, which are produced cheaply using standard fabrication techniques, consist of photodiodes connected in series on a silicon wafer, with two on-board electrodes to complete a circuit. Like a solar cell, when exposed to light, the light is absorbed and converted into an electrical current that is proportional both to the intensity of the light and the size of the photodiodes. ‘Essentially, if you want to do electrochemical high-throughput experiments, you just need to buy our Specs device, everything else should be commercially available already,’ explains Lin.
To demonstrate the versatility of the devices the research team carried out a series of experiments. The first was a reaction – a simple bromination – that they knew worked well to make sure they could calibrate the devices and ensure they were robust. They then validated the devices with other more challenging reactions published in the literature including oxidative, reductive and redox-neutral processes. ‘We just wanted to try all modalities of electrochemistry to make sure our Specs are compatible with the different types of electrochemical reactions,’ says Lin.
Then they looked at whether they could use the Specs to enable new chemistry by synthesising a library of 96 compounds with relevance to medicinal chemistry. ‘If you do drug discovery, you want to be able to make large library compounds and do biological screening with these new compounds,’ Lin adds.
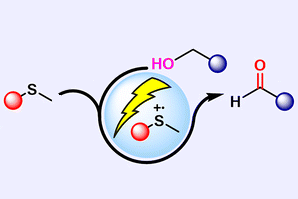
Finally, they used the Specs to develop two new reactions not recorded in the literature; the first formed carbon–nitrogen bonds by modifying a Shono oxidation reaction (typically used to form carbon–oxygen bonds), and the second to make sulfur- and nitrogen- containing compounds called sulfilimines, which have steadily gained attention in medicinal chemistry.
‘My student was able to run just three 96-well plates and he told me that within eight working days he was able to already fully discover this reaction, which is a pretty massive improvement; typically you probably have to do discovery over a couple months at least,’ says Lin.
Watch this space
The researchers anticipate that by simplifying the way electrochemical reactions are set up, Specs could reduce the barrier for the broad use of electrochemistry in organic chemistry. They are now in the process of commercialising Specs to make it available to the general organic chemistry community, hopefully later this year.
Shelley Minteer, professor of chemistry and director of the Kummer Institute Center for Resource Sustainability at Missouri University of Science and Technology in the US, tells Chemistry World she is ‘really excited’ about this work. ‘It’s basically using light to drive something wirelessly, which is really an ingenious and creative solution to this horrible wiring problem that we have in electrochemistry,’ she explains.
However, she says the Cornell team is potentially being too limiting by saying the device will reduce the barrier for the broad use of electrochemistry and organic chemistry. ‘I think it will be useful to fields outside of organic chemistry that are looking to use electrochemistry,’ Minteer adds. ‘Whether that’s sensor applications or energy applications, I think it will open itself up to people beyond just organic chemistry.’
References
B Górski et al, Nature, 2025, 637, 354 (DOI: 10.1038/s41586-024-08373-1)
















No comments yet