Stop counting steps and move on to some one-pot reactions
I recall a conversation with my industrial supervisor during my first week as a process chemist while he was explaining the background to my project. ‘Really? You made 100kg using this route? It’s fifteen steps long!’ I said, looking at the number of arrows in the scheme. ‘Well,’ he replied patiently, ‘we actually only isolated one intermediate, so in a sense it’s only two steps long’.
In this age of telescoping, scalable total synthesis and fierce competition, the definition of a chemical step – and therefore who has the shortest route to a target – can be controversial. New technologies such as biocatalytic cascades and flow chemistry make it easier than ever to avoid isolating intermediates, and chemists seem to squeeze more and more information over their arrows with every passing decade.
Ten years ago, as a student of total synthesis, I held strong opinions on this topic, but as a process chemist it’s now my job to blur the lines between steps as much as possible. You see, workups, isolations and purifications are incredibly resource and time intensive on scale. In addition, cleaning and checking reactors after use often takes as long as running the chemistry did. So, like undergraduates trying to cook a meal with single saucepan and fork, we strive to use as little equipment as possible in our pilot plant campaigns.
Of course we still worry about route length and efficiency, but operational complexity and equipment usage are major considerations too. We spend countless hours thinking about ways to run as many reactions as possible in a single reactor before an isolation or purification step is required. Unfortunately, this ability to consider the mutual compatibility of reaction conditions and the impact of oft-forgotten byproducts and spent reagents is a skill rarely taught outside of industry. Until recently, the norm for multistep synthesis in academia has been that every intermediate is isolated and purified unless such operations are impossible. But does this really make sense? After all, the best way to reduce time, cost and environmental impact for a synthesis is usually by eliminating workups and purifications, not by tinkering with reaction conditions.
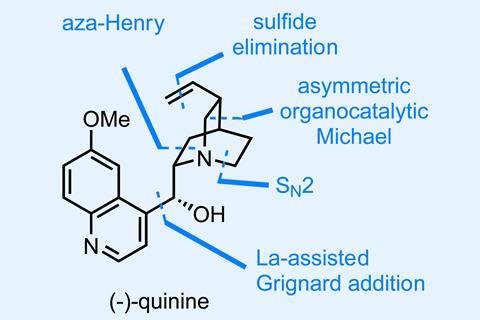
A recent total synthesis of the storied natural product quinine by Yujiro Hayashi and Takahiro Terunuma at Tohoku University in Japan does a good job of questioning this dogma by condensing a large number of chemical transformations into just five one-pot reaction sequences (accompanied by just five chromatographic purifications!).1
The first of these uses a combination of an organocatalytic asymmetric Michael reaction and an aza-Henry reaction to rapidly assemble a precursor to the molecule’s stereodense quinuclidine ring (figure 1). Cleverly, a sulfonyl amine is used as a precursor to the unstable Boc-imine used as the electrophile in this later step and elimination of nitrite and workup give the first isolated intermediate in good enantiomeric excess and yield.
The reactions in pot three are interesting (figure 2). First, there’s a neat one pot activation and elimination of the aryl sulfide that I hadn’t seen before. Classically, this is done via oxidation to the sulfoxide followed by strong heating, but these conditions are much milder. More impressively, these conditions allow this step to be coupled with a diisobutylaluminium hydride (DIBAL) reduction of the ester to the aldehyde – a finicky transformation that’s often hard enough without another reaction’s junk present.
From here, just two more one pot sequences complete the target. So how many steps is this synthesis in total? The authors don’t say, and I’m not going to venture an opinion. But I can say I would rather run this synthesis than any of the many other syntheses of quinine reported to date – and not just because I hate washing up.
References
1. T Terunuma and Y Hayashi, Nat. Commun., 2022, 13, 7503 (DOI: 10.1038/s41467-022-34916-z)













No comments yet