(–)-Scabrolide B (again!)
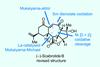
Proverbially, comparison may not bring joy – but it can be educational
Total synthesis is often likened to scaling an uncharted peak, so perhaps it’s not surprising that one of my favourite things to do outside the lab has long been rock climbing. In my preferred subdiscipline, bouldering, a climber attempts to struggle their way up 10–20 feet of rock with a lot of freedom (and minimal safety equipment). While this isn’t typically a team sport, it’s much more fun with a group of friends who can both assist with problem-solving and help soften falls.