(+)-Zincophorin methyl ester
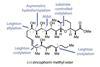
New and old reactions combine for an elegant and concise synthesis
In 1675, Isaac Newton wrote a letter to his friend Robert Hooke, asking for critique of his papers and making his now famous remark: ‘If I have seen further, it is by standing on the shoulders of giants.’ Almost 350 years later, this aphorism is no less true. This total synthesis of zincophorin methyl ester is a great example of a pragmatic and practical approach to a complex natural product that pulls together chemistry old and new