Karl Collins applauds the simplicity of adding silicon to heterocycles with KOtBu
While silicon is probably most familiar in organic synthesis as part of protecting groups, its utility extends much further. The Peterson olefination, Mukaiyama aldol reaction and Fleming-Tamao oxidation are just some of many well-established transformations.
Since the early 2000s, (hetero)aromatic silanols and silanes have begun to be used in metal-catalysed cross-coupling. Although boron reagents’ dominance in the classical Suzuki-Miyaura reaction is unlikely to fade away any time soon, the low cost and widespread availability of bulk silicon, and more recent murmurings about genotoxity of boron suggests that silicon-based building blocks may well yet have a future.
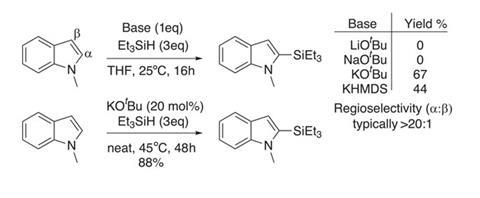
For this to become a reality, we need easy and cost-effective access to silylated aromatics. Traditional methods using metallated aromatics and silicon electrophiles have all the inherent limitations associated with strong bases, and generate stoichiometric amounts of waste. Catalytic methods using rare transition metals to silylate arenes and heteroarenes are emerging, and a collaboration between Brian Stoltz and Robert Grubbs at the California Institute of Technology, US, has taken steps towards a more ideal synthetic protocol.1
The team has developed simple protocols for dehydrogenative C–H silylation of numerous heterocycles with hydrosilanes, using catalytic potassium tert -butoxide as the only reagent. Initial optimisation studies using N -methylindole and triethylsilane identified that a potassium cation is crucial to mediate the reaction. The tert -butoxide salt seems to give the best reactivity and product yield (figure 1), and the team has successfully coupled several electron-rich N -protected indoles with a range of hydrosilanes.
Unfortunately, indole itself and electron-deficient systems are unreactive, ruling out many common nitrogen-protecting groups. Fortunately, benzyl, methoxymethyl acetal (MOM) and 2-trimethylsilylethoxymethyl (SEM) protected systems provide an opportunity to free the N–H.
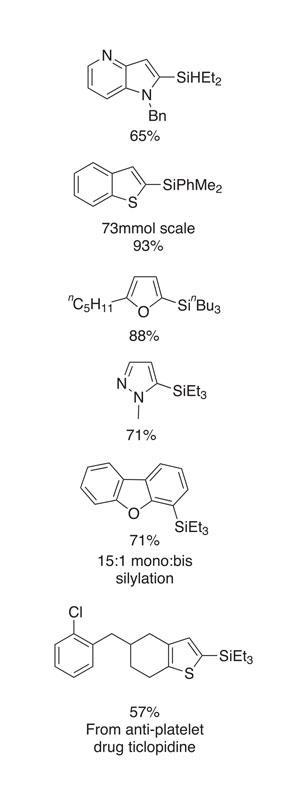
The reactions typically proceed effectively – although the substrate scope set out in the paper, and an intermolecular reaction evaluation, show only moderate functional group tolerance. That said, aryl chlorides, olefins and alkynes are useful groups that are tolerated, as are tertiary amines and pyridine-type nitrogen atoms, both of which are relevant in an industrial setting. The method is also applicable to a wide variety of heterocycles (figure 2). Amongst others, this included thiophenes, benzothiophenes, furans and N -alkylated pyrazoles.
The little bit of mechanistic speculation the team includes is also quite fascinating. They wisely make no allusions towards a C–H activation type mechanism,2 and their initial experiments suggest the involvement of a radical species. When the radical scavenger TEMPO is included, a TEMPO-SiEt3 adduct forms, suggesting the presence of a silyl radical. However, despite this observation, a simple (Minisci -type) radical substitution seems unlikely. Electron deficient heterocycles such as pyridine and quinoline – prime substrates for such reactions – fail.
Trace metal analysis and studies on a range of commercial and prepared KOt Bu sources seems to rule out traces of transition metals catalysing the reaction. The selectivity of the reaction (a vs ß) appears to rule out an electrophilic substitution mechanism, and the aromaticity of the compounds also seems irrelevant. The team suggests that all the evidence points to a distinct mechanism for functionalising heteroaromatic systems – it will be intriguing to see whether heteroaromatic radical species have a role to play.
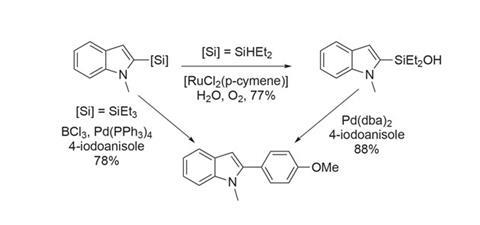
While there are limitations in terms of functional group tolerance and applications to more complex molecules, such facile access to silylated heterocycles is very useful. The products can be coupled with aryl halides directly or via the silanol (figure 3). The versatility of silanes in synthesis means that these products are not limited to cross-coupling chemistry, and their potential in organic materials and pharmaceuticals makes this work even more intriguing.
References
1 A A Toutov et al, Nature, 2015, 518, 80 (DOI: 10.1038/nature14126)
2 A Studer and D P Curran, Angew. Chem. Int. Ed., 2011, 50, 5018 (DOI: 10.1002/anie.201101597)












No comments yet