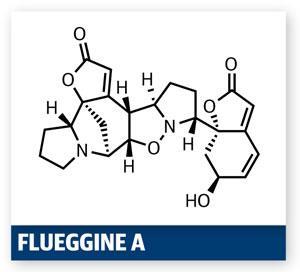
The structure and the synthetic challenge it poses also captured the fascination of a Chinese team led by Chuang-Chuang Li of Peking University, Shenzhen. The group targeted several molecules in this potent family in a recent paper,1 but it is their exceptionally short synthesis of flueggine A that really grabbed me. Key to the brevity of the synthesis was recognising that the isoxazolidine functionality could act as a keystone in this target, a conclusion also suggested in biogenesis studies.2 Considering this retrosynthetically, the team realised that the target is pseudo-symmetric – a dimer of similar (but not identical) halves, and that the isoxazolidine was the glue between them.
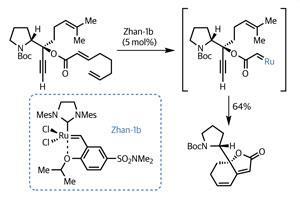
Working asymmetrically from the off, the team began their synthesis with the organic chemist’s favourite amino acid, proline. Four relatively high-yielding elaboration reactions installed several sites of of unsaturation, including an acetylene and a trio of alkene groups. The team then ambitiously treated this intermediate with a ruthenium-based catalyst, knowing that some metathesis action was likely to ensue – but with a high potential for competing reactions (figure 1). Remarkably, the team achieved their goal of a relay ring closing metathesis, and in an impressive yield, building a 5,6-fused bicycle featuring a rather sensitive dienoate system.3
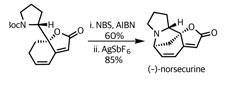
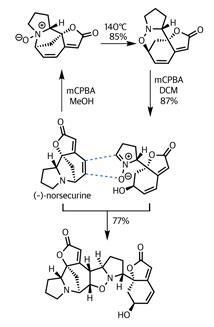
This was exactly what the team had in mind. Adding their remaining (–)-norsecurine (an equally potent dipolarophile) and tickling with a little heat prompted the pericyclic reaction – forming the remaining isoxazolidine ring and uniting the fragments.
Again, selectivity was key to success in this reaction. Not only are there several side reactions that could have competed, there are also several potential orientations of reaction. However, the gods of stereoelectronics were clearly shining on the team, as they isolated flueggine A in excellent yield.
Impressively, the team did not simply call it quits at this point – they went on to complete the synthesis of another intricate member of this family of natural products, (+)-virosaine B. This only underscores the utility and practicality of their route into this series, as well as the value of dipolar cycloaddition reactions in the synthetic toolkit.
Paul Docherty is a science writer and blogger based in Reading, UK












No comments yet