Newly-independent research groups often bring new perspectives to synthesis
New team members often bring new perspectives and can open up completely new avenues of thought. The same can often be said of young academics looking to find a niche and make their mark, as they face up to the challenge of moving into a community of established experts who are not always overly receptive to the new guy on the block. As a result, it is worthwhile seeking out new research groups to look for that new perspective born of a need to succeed, and without the constrictions about what they ‘know’ and where their expertise lies. Sometimes it takes a little effort to identify new researchers as they build upon their ideas, but others grab your attention straight out of the block, as is the case with Andrew McNally of Colorado State University, US.
The McNally group’s first independent work is a very attractive – and apparently simple – two-step protocol for selectively functionalising the C4 position of pyridines with alkoxide nucleophiles.1 This is by no means a trivial transformation and has plenty of potential for real world application. McNally begins by identifying C4 phosphonium salts of pyridines as suitable intermediate substrates. These rarely exploited salts were first investigated in 1987 by Ernst Anders at the University of Erlangen-Nuremberg, Germany, and can be formed from the unfunctionalised parent pyridine with essentially complete selectivity.2
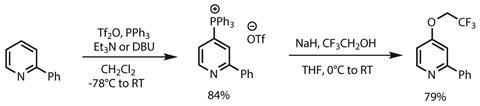
The team has prepared several phosphonium salts, adding triphenylphosphine directly to pyridines in dichloromethane (figure 1). The pyridines must be activated with trifluoromethylsulfonic anhydride (Tf2O) in the presence of an organic base, and the team suggests that the excellent selectivity is driven by optimal orbital overlap rather than any steric factors preventing C2 functionalisation. The phosphonium salts are isolated without chromatography as stable and easy to handle solids, though digging around in the supporting information shows that isolation isn’t quite as simple as crashing the salts directly out of the reaction mixture, and can require a fair bit of tinkering. In a second step, adding the phosphonium salts to sodium alkoxides in tetrahydrofuran at 0°C forms carbon–oxygen bonds on warming to room temperature.
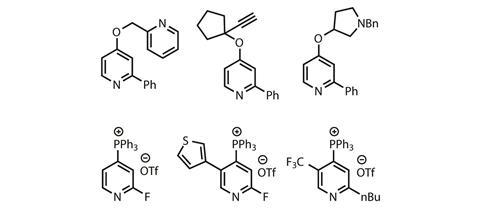
The variety of pyridines exhibited in the paper won’t blow anyone away, but important substrates such as C2-halogenated pyridines pose no problems (figure 2). The reaction tolerates electron-donating and -withdrawing groups at the C3 or C5 positions, and steric bulk doesn’t appear to be a major challenge, although no 3,5-disubstituted pyridines are reported. The phosphonium salt formation is generally high yielding and the alkoxide substitution is typically above the 60% mark. Pyridines that are already C4-substituted react next to the nitrogen atom as would be expected, and simple pyrimidines and pyrazines are also viable substrates. The alcohol scope is apparently unrestricted with respect to steric bulk, with primary, secondary and tertiary alcohols all successful.
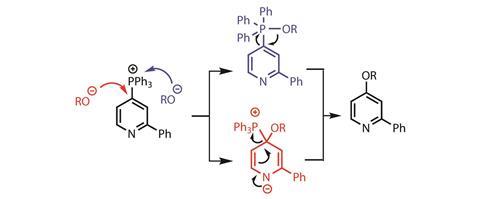
Presuming that steric interactions do not significantly affect C–O bond formation leads me to support the researchers’ favoured mechanistic proposal: the alkoxide attacks phosphorus to generate an alkoxyphosphane intermediate, followed by intramolecular ipso-substitution (figure 3). Although the team cannot categorically rule out a nucleophilic aromatic substitution, it is hard to imagine direct addition of a hindered tertiary alcohol to such a sterically encumbered carbon atom. That said, preliminary experiments demonstrate reaction of the phosphonium salts with a thiolate, sodium azide and lithiated heterocycles giving the feeling that, mechanistically, the phosphonium salts’ behaviour is more complex than is initially apparent. The team finishes with a typical late-stage functionalisation of relatively simple pharmaceuticals, but is missing a truly relevant functionalised example to get industrial chemists straight in the lab.
McNally’s team has made an impressive statement as a new independent group. They have set the bar suitably high for themselves with detailed and honest supporting information, although it would have been useful to define the limitations of the reaction. A word or two on alternative ways to isolate the phosphonium salts would also have been a great addition.
References
1 M C Hilton, R D Dolewski and A McNally, J. Am. Chem. Soc, 2016, 138, 13806 (DOI: 10.1021/jacs.6b08662)
2 E Anders and F Markus,Tetrahedron Lett., 1987, 28, 2675 (DOI: 10.1016/S0040-4039(00)96178-1)










No comments yet