Ketones perform at the palladium
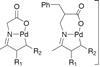
C–H activation takes the stress out of organometallic couplings
Jin-Quan Yu and his team continue to make outstanding contributions in the field of C–H activation. For example developing an increasingly general β-arylation of ketones that breaks the dogma of perceived conceptual limitations.
