Reformatskii reaction
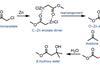
The survivor of a once-great empire of zinc chemistry
Six hundred miles east of Moscow, at the very edge of the European–Asiatic divide is the University of Kazan which, by the mid-nineteenth century, had emerged as Russia’s pre-eminent chemistry department. It was here that Sergei Reformatskii would discover the reaction that now bears his name.