Easy to use and robust, the Evans or JM balance has been on the market in various forms since 1974
The limerick, that most contrived and English of verse forms, is often used to highlight the eccentricities of life and, especially, of people. The writer and painter Edward Lear published scores of ridiculous rhymes to the delight of generations of both adults and children. That he should have been English is not surprising: England is one country where eccentricity is not just accepted but prized, and if it is often associated with wealthy aristocrats, examples abound among artists and scientists.

Dennis Evans was considered an eccentric. He came from a working class background and studied chemistry at the University of Oxford. An outstanding student, his almost photographic memory was the stuff of legend. In his second year, Evans, who had read Linus Pauling’s The nature of the chemical bond, attended Pauling’s lectures at Balliol College. He corrected Pauling several times, mid-lecture, for getting values of physical measurements wrong. Later, Pauling would just turn to Evans each time he needed a value.
Working with Rex Richards, a young lecturer with interests in magnetism and a pioneer in the chemical applications of NMR, he built a sensitive micro-calorimeter to study phase transitions of quinol (benzene-1,4-diol). His observation that the b form of quinol effervesced in water led to the discovery that it was a clathrate - with air trapped inside the crystal lattice. Evans carefully crystallised quinol clathrates of dioxygen and nitric oxide at high pressures, and then used the Gouy method to measure their magnetic properties.
After a short postdoc at the University of Chicago, US, with Robert Mulliken, he was appointed to a lectureship at Imperial College London in 1955. Evans began using the unpaired electrons of oxygen, at high pressure, to perturb the energy levels of aromatic hydrocarbons. Under these conditions, benzene turned yellow. In one memorable - but unpublished - experiment, acetylene and oxygen at 150atm exploded just as Evans was releasing the pressure. He escaped almost unscathed; the apparatus was another matter.
But Imperial’s purchase of an NMR spectrometer gave Evans the opportunity to build on his magnetic studies; he realised that paramagnetic impurities shifted NMR signals in predictable ways. Turning this round, he showed that a sealed capillary of pure solvent inserted into an NMR tube of a solution of a paramagnetic compound gave a spectrum with two peaks for the solvent, their frequency separation dependent on the concentration and magnetic moment of the solute. The Evans NMR method, a boon to organometallic chemists, was born.

But the Gouy method – weighing a cylindrical sample with and without a field – in spite of its fiddliness, persisted as the method of choice for room temperature magnetic studies, especially for insoluble materials. In 1967, Evans turned the experiment on its head. Intrigued by a torsional balance developed in 1937 by British physicist Alexander Rankine, Evans built a device where the sample was fixed; it was the magnet - glued to the pan of a balance - that was weighed. What it lacked in precision, it gained in robustness and low cost, and it could be used for tubes fitted with valves for air-sensitive compounds. The idea just needed honing.
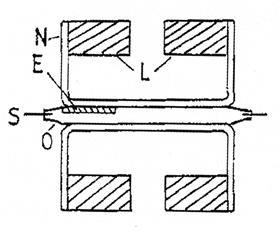
Eight years later, and with a little help from specialist metals company Johnson Matthey, came its successor. Two pairs of magnets (L) were glued between the arms of an H-frame (N). The sample sat in one gap, a small coil in the other. The entire assembly pivoted horizontally around a torsion strip (S). When a sample tube was placed between one pair of magnets, the torsional force was restored by the current in the coil between the other pair, giving a reading on a display. Easy to use and robust, the Evans or JM balance has been on the market in various forms ever since. Ironically, in these impact-obsessed days, at time of writing the original paper has been cited a mere 12 times.
But Evans was not just interested in electrons. His sometime pets included an alligator and a variety of arthropods. He was fascinated by the recreational aspects of organic chemistry, synthesising psychoactive molecules that he tested on himself and his friends. He once shared his flat with Christine Keeler, of Profumo scandal fame, and mixed with artists and poets such as William Burroughs and Bridget Riley. His political views were strongly left of centre, and he had several run-ins with authority.
But in the lab he was a chemist’s chemist. And how better to celebrate him, than with a limerick?
Faraday first noticed the pull
That Gouy exploited in full.
But Rankine’s thin wire
Would Evans inspire
Four magnets to balance and null.
References
D F Evans, J. Phys. E: Sci. Instrum., 1974, 7, 247(DOI: 10.1088/0022-3735/7/4/007)
Thanks are expressed to Malcolm Green and Bill Griffith for assistance with this article












No comments yet