Add more peroxide to stabilise new drug molecules
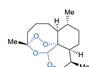
From a curiosity to life-saving medicine – how peroxides make for stable and efficient drugs

From a curiosity to life-saving medicine – how peroxides make for stable and efficient drugs