Testing small amounts of blood for the presence of disease markers could revolutionise how we detect cancer. Clare Sansom reports
-
Early and precise diagnosis: Liquid biopsies, which analyse blood for disease markers, offer a non-invasive method to detect cancer early and precisely, potentially transforming cancer from a killer disease to a manageable one.
-
Circulating tumour cells (CTCs): These cells, shed by solid tumours into the bloodstream, can be analysed to provide real-time information about the tumour’s aggressiveness and metastatic potential, aiding in prognosis and treatment selection.
-
Technological innovations: Techniques like automation, genomics, next-generation sequencing, and AI are crucial for decoding the complex signals from liquid biopsies, although standardisation and validation in large patient cohorts are still needed.
-
Clinical applications and challenges: While promising technologies like the Parsortix system and Dxcover’s multi-cancer platform show potential, extensive clinical studies and international collaboration are necessary before these methods can be routinely used in clinical practice.
This summary was generated by AI and checked by a human editor
The charity Cancer Research UK recently described the disease as ‘the defining health issue of our time’. Perhaps they would say that, but even in the age of Covid it is undeniably one of the most important issues. Many cancers are diseases of old (or at least middle) age, and as the population ages, it is becoming even more widespread. Half of us are now predicted to develop cancer during our lifetimes and, globally, one person is diagnosed with a form of the disease every one or two seconds. And even this doesn’t account for the hidden burden of undiagnosed disease, particularly in low- and middle-income countries.
As late as the mid-20th century, few people diagnosed with cancer could expect a complete cure. Today, however, 90% of cancers are curable if they are diagnosed early enough, but that early diagnosis is critical. So, too, is precise diagnosis: a disease that would, 75 years ago, have been described only as breast or lung cancer can now be assigned a specific molecular profile leading to a detailed prognosis and, more importantly for the patient, a personalised treatment plan. When we get this right, more and more types of cancer will be turned from killer diseases into curable or at least manageable ones. Yet, as Cancer Research UK’s recent strategy paper highlights, we in the UK are right at the bottom of the league of rich countries for cancer survival rates: and this is largely because we fail to diagnose it early or precisely enough.
One of the most common techniques for detecting cancer is the biopsy, extracting a sample to test for disease. At least historically, biopsies for cancer involve taking samples of tumour tissue: either excisional (removal of the whole tumour) or incisional (removal of a sample). If cancer is only suspected, however, clinicians and patients alike will be keen to avoid invasive procedures. The concept of a liquid biopsy for cancer – sampling body fluids, usually blood, and analysing their components as a proxy for sampling the tumour itself – was introduced in 2010 by Catherine Alix-Panabières of the University Medical Centre of Montpellier, France and Klaus Pantel of the University Medical Center of Hamburg, Germany: initially through sampling circulating tumour cells (CTCs) in blood. These cells, however, had been known of for a long time: they were first described by Thomas Ashworth in 1869.
Blood biomarkers
Circulating tumour cells are the small proportion of cells that solid tumours shed into the bloodstream and are carried round the body in the circulation, either as isolated cells or in clusters. They can act as ‘seeds’ for distant metastases; generally, the more aggressive and invasive a tumour is, the more cells it will release into the bloodstream. Often, they will carry molecular markers for metastatic disease that are absent from the cells of the original tumour, and identifying these markers on CTCs via a blood biopsy can help clinicians keep one step ahead of the developing disease.
Many other types of molecule can be extracted from biopsies of blood – or indeed other body fluids – to yield information about a solid tumour. Most of these are tumour components: proteins, metabolites and cell-free circulating tumour DNA (ctDNA) in the blood. Biomarkers that are induced by a tumour rather than derived from it, such as immune cells and cytokines, are particularly useful in the early stages of disease or in the context of immunotherapy.
Solid tumours release CTCs, ctDNA and other molecules into the bloodstream constantly and many of these have short half-lives, so a blood biopsy, unlike a tissue biopsy, can provide information essentially in ‘real time’. Alix-Panabières believes that blood and other liquid biopsies are ‘cancer-dependent and stage-dependent’ and can be used to derive a very precise, dynamic picture of a tumour with great clinical value for prognosis prediction and treatment selection.
Of course, obtaining and purifying sufficient quantities of cells or molecules from a liquid biopsy is only the first stage. A list of the companion techniques that are being developed to decode analyse the resulting signals reads like a roll-call of innovative and important technologies in healthcare today: automation, genomics, next-generation sequencing and artificial intelligence.
It is perhaps surprising that there are as yet no blood biopsies for cancer in clinical use other than tests for specific mutations. Alix-Panabières set out the path forward in an influential Nature article early in 2020 but disappointingly little has changed in the four years since. ‘Before any technology is registered and used in routine clinical practice it must be validated in large cohorts of patients, with well-defined inclusion criteria,’ she explains. ‘We still need to define standardised protocols for the assays and reliable algorithms to derive a precise clinical profile from the various circulating biomarkers’. The clinical studies that are needed to achieve these aims are large and extensive and are run most effectively by international consortia such as those set up through the European Liquid Biopsy Society.
Analysis of CTCs from a blood biopsy is complicated by the fact that they only form a tiny proportion of the cellular component of the blood: looking for tumour-derived cells to analyse is like looking for a needle in a haystack. Before these cells can be extracted from a whole blood sample, that sample must be enriched; ie, the proportion of these cells must be increased. The FDA-cleared technology CellSearch, which is robust and reproducible, was validated two decades ago to detect CTCs and clusters in metastatic breast, prostate and colon cancer. Using CellSearch combined with the DEPArray, it is possible to assess tumour heterogeneity at the single cell level without any leukocyte contamination.

Another promising technology has been developed by Angle, a company based in Guildford, UK. This system, known as Parsortix, captures and harvests CTCs from a sample of whole blood. It is a microfluidics system that filters cells based on their physical properties alone, independent of any molecular markers of cancer, and can therefore be used to harvest cells from any solid tumour. It is based on a cassette with a critical gap of 6.5µm through which the blood flows. Red blood cells are small enough to flow through this gap and into the device’s waste channel, and the larger white blood cells can be compressed enough to squeeze through. Circulating tumour cells, however, are less easily deformed, so isolated CTCs as well as CTC clusters will remain inside the cassette. Residual normal cells and debris are rinsed away, and the remaining CTC-rich fraction is eluted from the cassette for analysis.
‘The Parsortix PC1 system is the first device to be cleared by the FDA for the capture and harvest of CTCs from metastatic breast cancer patient blood,’ explains Angle’s Beatrix Thompson. ‘This is an important indication because the metastases, and the CTCs that they are derived from, are intrinsically different from the primary tumour at the molecular level.’ Oncologist Julie Lang, formerly of the University of Southern California and now based in Cleveland, led a consortium that tested PC1-harvested CTCs as a surrogate for standard tissue biopsies in this indication. ‘Breast cancer is a very heterogeneous disease,’ she explains. ‘Some subtypes are very aggressive, with no targeted treatment options available; in contrast, hormonally sensitive cancers have an excellent prognosis but may still recur and metastasise after perhaps 20 years of follow-up.’ The consortium compared gene expression in the harvested CTCs with that in tumour cells obtained in a standard tissue biopsy and found similar expression levels of those genes that were best able to predict treatment response. ‘Our benchtop liquid biopsies use a tiny volume of peripheral blood – only 7.5ml – and the system is very quick; it only takes a couple of hours to obtain useful results from a single blood sample,’ she adds. ‘But we need to validate the system in much larger studies before it can be routinely used in the clinic.’
Assays and algorithms
A diagnosis of a brain tumour is often one of the most devastating that a patient can receive. These tumours are more likely to affect young and middle-aged people than many other cancers and some have very poor prognoses. Early diagnosis again can change some prognoses dramatically, but it is one that is difficult to make with any certainty. The early symptoms are diffuse and mimic many other, much commoner conditions: if everyone who attends primary care complaining of a headache were to be sent for a brain scan, a few more cancers would be caught early but at the expense of bringing the system close to collapse.
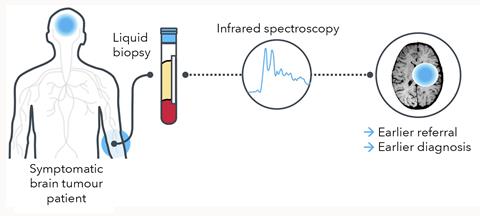
This is a clear case where a non-invasive blood test to identify those patients most likely to benefit from a scan can make a huge difference. And this was the first indication tackled by Dxcover, a startup company based in Glasgow, Scotland, around the ground-breaking research of Matthew Baker at the University of Strathclyde. ‘We are developing a multi-cancer blood biopsy platform that involves the infra-red spectroscopic analysis of dry blood serum plugged into an AI algorithm,’ explains Baker. Dxcover’s brain tumour assay is best thought of as a triage process, identifying those patients most likely to have cancer to be fast tracked for urgent MRI scans.
The principle behind Dxcover’s assay is a very simple one. A tiny amount of serum derived from the blood of a patient or volunteer is applied to three of the four wells of a prepared slide, with the fourth well left clear to give a background measurement. Once the samples have dried, each well is scanned three times with broad-spectrum infra-red light for FTIR analysis. ‘Most assays will concentrate on a single type of molecule, perhaps DNA, proteins or metabolites,’ says Baker. ‘In contrast, our system will pick up a signal from anything that is there, so this is genuinely a multi-omics approach’. Disentangling the signals from different types of molecule is tractable because they tend to absorb infra-red light at distinctly different sets of frequencies.
A cancer can be thought of as a complex organ just like any other, with a distinct molecular profile. Once a set of spectra have been obtained from a blood sample, they are plugged into an AI algorithm to predict how far they match the profile of a brain tumour, or any other cancer type. And this focus on multiple types of molecular marker is of particular value in the crucial early stages of the disease. ‘At first, tumours are small and slow-growing, and they slough relatively small quantities of DNA into the bloodstream,’ adds Baker. ‘Those assays that focus only on circulating tumour DNA [as opposed to other tumour- or immune system-derived molecular types] cannot produce a strong signal until the tumour grows.’
Sensitivity vs specificity
A US consortium led by oncologists Dan Landau of the New York Genome Center and Adam Widman of the Memorial Sloan Kettering Cancer Center in New York has developed a blood biopsy that combines genomics with machine learning to detect cancer recurrence almost in real time. This assay does use circulating tumour DNA, despite its initially very sparse signal. ‘You can think of a tumour shedding DNA as a leaky tap,’ explains Landau. ‘Initially, very little of the DNA in the bloodstream comes from the tumour, but even then our deep gene sequencing can pick out one tumour-derived DNA molecule in tens or even hundreds of thousands.’ Machine learning is used to distinguish sequence patterns indicating mutations found in cancer cells from those that suggest an artificial cause such as an error in DNA sequencing.
Landau and his team have been working on this technique since 2017, but it has only become clinically relevant very recently as the cost of sequencing has decreased and the power of machine learning increased. They are now testing it in many different solid tumour types and clinical indications, from screening to the detection of residual disease in patients in remission. ‘There is nothing in our method that makes it specific for one or more tumours, but the algorithm will need to be trained to recognise the specific types of mutation most closely associated with each one,’ adds Landau. An algorithm for melanoma could be focused on types of DNA damage that are often caused by ultraviolet light, and one for lung cancer on damage caused by cigarette smoke.
With this approach, the trade-off between sensitivity and specificity, between false negatives and false positives, is crucially important and it depends on the indication. If you are screening a population of apparently healthy individuals for a rare disease, it is probably sensible to minimise the distress and expense caused by false positives. If, on the other hand, you are monitoring recovered cancer patients for the first signs of recurrence, it is best to minimise false negatives even if a few patients have ‘recurrences’ picked up unnecessarily. Another difference is the amount of blood needed: as the ‘leaky tap’ starts shedding cells slowly, a larger blood volume is needed to pick up a signal in screening applications than in monitoring recurrent or metastatic disease.
These case studies show how technologies such as genomics and AI can extract enough information from a simple blood sample to detect, diagnose and potentially transform the prospects for patients with a wide range of cancers at different stages. Progress towards registration for clinical use is still slow, however, and many more wide-ranging studies are needed before the technique can be routinely available in oncologists’ clinics, let alone in the family doctors’ surgeries where screening and monitoring could have the greatest benefit. Cancer Research UK is calling for a greater emphasis on community-based diagnostic services: supporting these extensive clinical studies would be an excellent use of their funds.
Clare Sansom is a science writer based in Cambridge, UK
Article update 01 October 2024 to remove inaccuracies about Dxcover’s algorithm
















No comments yet