Raman spectroscopy has been seen as a tool for physicists and chemists but Hayley Bennett finds it has the potential to cause a major shift in the way we do medicine
Chandrasekhara Venkata Raman, a physicist at Calcutta University in India, had spent seven years studying light. Initially, he’d hoped to revise theories about why the sea looks blue but soon realised he’d stumbled on something potentially more interesting. In March 1928, he stood in front of members of the South Indian Science Association in Bangalore, India, and pointed out lines on grainy spectrograms. These unremarkable images of white lines on black backgrounds showed what happened to coloured light when he shone it through liquid benzene – it changed colour a little bit.
The spectrograms represented the first public airing of what was to become known as Raman scattering of light or the Raman effect. There would be implications across the physical sciences, Raman told his audience in Bangalore – for radiation and wave-theory, x-ray optics, thermodynamics and chemistry. ‘We are obviously only at the fringe of a fascinating new region of experimental research,’ he said. ‘It all remains to be worked out.’ What he didn’t foresee, however, were the implications for medicine.
Having tested at least 60 different liquids and some gases, Raman and his student Kariamanickam Srinivasa Krishnan were persuaded that the scattering they were seeing was a universal phenomenon and called it ‘a new radiation’. But it turns out that blood, tooth and brain cells scatter light in this way too and today’s Raman spectrometers are increasingly being used to study the molecular make-up of human tissue.
We are only at the fringe of a fascinating new region of experimental research
CV Raman
The Raman effect occurs because of molecular vibrations in the material that take energy from, or give it to, photons of light, causing a change in wavelength or colour. Importantly, the nature of that change is unique to the molecules inhabiting the material; it can be thought of as a chemical fingerprint. Because Raman spectroscopy can pick out very small chemical changes, it allows biomedical researchers to spot even minor fluctuations in the molecules associated with disease and categorise tissues according to whether they are healthy or not.
This is achieved in spite of the fact that Raman is ‘fundamentally a weak technique’, explains Karen Faulds, a Raman expert at the University of Strathclyde in Scotland. Raman scattering was barely detectable back in the 1920s because such a small portion of the incoming light behaves in this way; only one in a million or even 10 million photons. Raman and Krishnan had to focus sunlight through a 180mm telescope to get a strong enough beam for their experiments. According to Faulds, we’re only able to get better signals today due to a raft of technological advancements: ‘The instrumentation, the detectors, the lasers, the filters and so on.’
Shift happens
A modern Raman spectrum looks very different to the images – like inverted barcodes – that Raman showed in Bangalore. Raman and Krishnan exposed two photographic plates for each material – on one, the bands represented the wavelengths of light in the beam entering the material and, on the other, those in the beam exiting it. Then they simply compared them, looking for changes in wavelengths, or ‘shift’.
A modern spectrum shows only the shift, as a series of peaks that corresponds to the wavelength changes. This pattern identifies the atoms and molecules in a material and tells us something about how they’re organised. Many biomedical researchers are using this approach to study biopsies or serum samples, while others are working with living tissues in animals or humans.
According to Faulds, Raman researchers are now starting to find ways for their techniques to fit into clinical pathways. So what diseases can you detect using a Raman spectrometer? More than you’d think – the diagnostic power of Raman seems to know no bounds, in fact. A group led by Luis González-Solís at Centro Universitario de los Lagos in Mexico, for example, tested Raman for diagnosing tooth decay, type 2 diabetes, and breast and cervical cancer, and for monitoring leukaemia treatments, just in the last four years. Another group at the Massachusetts Institute of Technology (MIT) in the US is developing Raman-based technology for guiding placement of epidural needles and continuous blood glucose monitoring for diabetics.
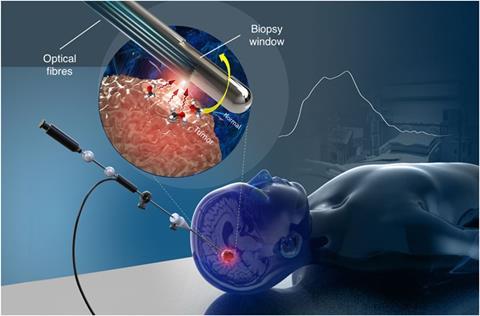
MIT’s needle placement technology, now being developed by spin-out company Medisight, provides perhaps the most straightforward example of how Raman can be applied to differentiate between one human tissue and another. As Jeon Woong Kang of MIT and Medisight explains, an epidural is a common procedure relying almost entirely on clinician experience. It involves inserting a 10cm-long needle into a 2mm space between two layers of tissue that are hard to identify; unsurprisingly, this can result in errors. ‘About 10–30% of needles are going into the wrong tissue and causing complications,’ says Kang. ‘People think epidural is a very, very safe procedure because it’s so common… but even in the US, each year, several cases are happening of death or spinal cord injuries. So what we developed is an intra-needle Raman sensor, which goes inside the needle and as we are inserting it, we identify tissues at the needle tip in real-time.’
In 2016, the group tested their needle sensor in tissues dissected from pigs. They used machine-learning algorithms to classify the types of Raman spectra they got from the various tissues close to the spinal cord. As their technique shows, it’s not crucial to know the details of the molecules within each tissue, as long as a machine can distinguish between them. The researchers found that the Raman spectrum for every one of the eight layers, from the outermost epidermis/dermis to the innermost spinal cord, had a unique signature. Two other techniques – diffuse reflectance and fluorescence spectroscopy – couldn’t fully differentiate.
Similarly, the technique that González-Solís’ group used in 2018 to discriminate between serum samples from healthy volunteers and those with type 2 diabetes employed a statistical method often used in pattern recognition and machine learning. Visually, the diabetic pattern does appear slightly different to the typical healthy Raman spectrum if the two are overlaid, but the differences are minor enough to suggest a machine would be needed to spot them every time.
Kang’s group is also getting into the busy diabetes space, where their portable Raman sensor promises continuous glucose monitoring via a wrist cuff; instead of taking glucose measurements from blood samples, it senses concentrations from outside the skin. Whilst ‘minimally invasive’ sensors that in principle do a similar job are already on the market, the patient still has to insert a wire, Kang points out. ‘The good thing is that it’s continuous monitoring, but the bad thing is that it’s not too accurate and even though it’s minimally invasive – still, it’s invasive,’ he says. His own Raman-based, non-invasive system promises more accurate readings and about the same number of regular blood tests required to recalibrate the sensor.
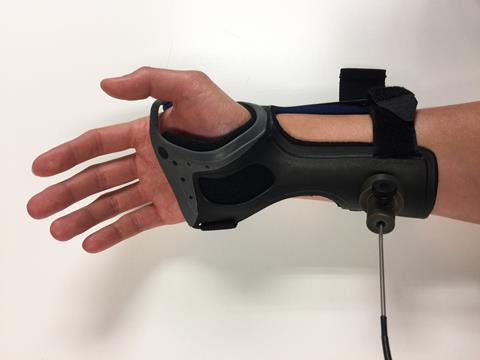
Both Kang and the Mexican team’s study single out the same key advantage of using Raman: it isn’t affected by water, which is an important feature when you’re studying human body fluids and tissues that are practically made of the stuff. Near infra-red spectroscopy (NIR), another potentially useful non-invasive technique, suffers from this problem. It’s very sensitive to the vibrations of water molecules, and the signals of water and glucose overlap. Thus, despite NIR producing a much more intense signal than Raman, both teams suggest it is essentially useless for glucose monitoring because of water interference.
Shifting to cancer treatment
If diabetes diagnosis and monitoring represent a huge potential market for Raman, then cancer diagnosis and surgery could be an even bigger one. Kang is currently developing a tissue scanner that differentiates between cancerous and non-cancerous tissue using a combination of different spectroscopic techniques, one being Raman. Meanwhile, Kevin Petrecca, a neurosurgical oncologist at McGill University in the Canadian city of Montreal, recently used Raman during brain surgery to differentiate between cancerous and non-cancerous tissue. His eventual aim is to help save healthy parts of the brain when removing a tumour. ‘That was really nice and I can see that being used,’ says Faulds.
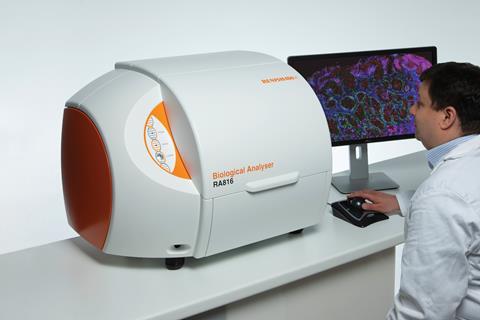
UK instrument-maker Renishaw, which makes Raman spectrometers for a wide range of applications, is also collaborating with brain surgeons. Working with trainee neurosurgeon James Livermore at the Oxford Radcliffe Hospital in the UK, they’ve taken tissue directly from tumours called gliomas and analysed them with their Biological Analyser instrument. ‘We installed this machine right next to surgery and as the surgeon was taking out brain tissue from the patient, we took some of that tissue and analysed it on our system,’ explains Martin Isabelle, senior applications scientist at Renishaw. ‘So we were able to give spectra then and there.’ Usually, gliomas are classified into different genetic sub-types by DNA sequencing and antibody-based testing of the tumour tissue, and different sub-types might require different treatment plans. The testing can take days, however. The Raman system, on the other hand, could provide an immediate indication of the sub-type based on the biochemical profile of the tumour. The intention, says Isabelle, is to move towards a fibre-optic Raman probe that could be used by surgeons, giving them information about the tissue they’re operating on in real-time.
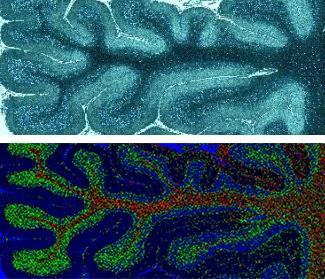
However, there are compromises to be made when taking Raman from the bench to a handheld probe. The challenge is miniaturising the components without reducing the already weak Raman signal to an undetectable level, or a level that would be useless for detecting small changes in tissue chemistry. In addition, using fibre optics introduces materials that produce their own Raman signal, potentially overwhelming the signal from the tissue. ‘It’s a real trade-off and it’s what’s stopped the progression of fibre optics in surgery,’ says Isabelle. So while the McGill group did manage to use a fibre-optic Raman probe in brain surgery, Isabelle says the quality of their spectra was ‘not as great as they’d hoped’, meaning they had to do some elaborate data processing. There are similar challenges ahead for Kang’s glucose sensor, which needs to be shrunk to a size that can be comfortably worn if the technology is to take off.
From animals to humans
There are ways to boost the weak Raman signal, though. One variation is surface enhanced Raman spectroscopy (Sers), which is based on the fact that putting a material on a metal surface, such as a gold nanoparticle, can amplify its signal – by more than a million times. This is the technique that Faulds’ team is using to detect biomarkers for different diseases. The signal that they enhance is provided by a Raman ‘reporter’ molecule on the surface of a nanoparticle. This whole entity is then delivered to a cancer cell or a bacterium, for instance, with the help of a targeting molecule such as an antibody that can latch onto the relevant biomarkers. It’s a technique that could provide a wealth of information about any given disease by detecting multiple biomarkers simultaneously. Renishaw has been testing a similar approach in collaboration with the Singapore Bioimaging Consortium, for detecting cancer markers in liquid serum samples taken from patients. But Faulds’ group wants to go further. ‘What we’re really pushing for now is to do detection in vivo,’ she says.

Right now, in vivo means animal studies. Faulds is one of team of researchers using Sers to detect the signs of heart disease in live mice that have received human tissue grafts. Their study is due to be published soon , but essentially, they injected the mice with modified gold nanoparticles targeted to inflammation markers and then shone a Raman laser on them. They used the information from the Raman spectra to produce an image mapping three different markers – a so-called ‘multiplex’ approach.
So could Sers work in human tissue too? ‘It’s kind of far off, but it’s where we want to move towards,’ says Faulds. In combination with another Raman technique – spatially offset Raman spectroscopy (Sors) – which detects signals from deeper within a tissue, it could provide unprecedented detail in diagnosing diseases where there are several well-established markers. However, there are plenty of hurdles to surmount, like the toxic nature of the nanoparticles and making the probes sensitive enough for deep detection. And as Isabelle points out, it’s a much more elaborate set-up than conventional Raman, which is simply shining a laser on the tissue and seeing what comes back. The beauty of Raman has always been that it’s non-invasive and label-free.
There’s also the path to registration that has to be navigated for new medical devices. Under the scrutiny of the regulatory approval process, even conventional Raman poses a risk because it uses lasers, which have the potential to damage eyes.
According to Faulds, the applications that actually make it will be those that could really make a difference to clinicians. ‘Historically, you know, a Raman spectroscopist was always telling a clinician “We’ve got this great technique, you need to use it!”’ Faulds laughs. ‘And they would say “Hmm, well, that won’t really change anything.”’ But she thinks that approach is disappearing now and spectroscopists are more interested in collaborating with clinicians to figure out techniques that will help them. What’s still needed from the clinical side, says Isabelle, is greater awareness of Raman’s promise and, in some cases, acceptance that there might be better ways of doing things.
We’re now a long way from that simple line spectrum presented by Raman himself in Bangalore. While the fundamental physics of his discovery remain the same, the implications have been sown far and wide. 90 years on, Raman spectroscopy may still be viewed as a ‘weak’ technique but it’s poised to have a powerful influence on the way we deal with disease.
Hayley Bennett is a science writer based in Bristol, UK











No comments yet