The chemistry of the microbiome
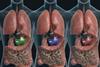
Our gut bacteria are carrying out chemistry on our behalf, but without us knowing much about it. Now, scientists are starting to examine their enzymes
Our gut bacteria are carrying out chemistry on our behalf, but without us knowing much about it. They can be part-metabolising drugs as well as processing our food into things that help or harm us. Scientists are starting to examine their enzymes, find out what they’re up to, and see if they can be harnessed to help us