A gold cluster catalyst developed by researchers in China can selectively hydrogenate nitrobenzene to make p-aminophenol. The reaction proceeds through a previously unidentified mechanism that is completely distinct from typical industrial routes.
p-Aminophenol is an important intermediate for preparing paracetamol – the most prescribed drug globally – and other drugs. Current methods for producing p-aminophenol often require multiple synthetic steps and complex purification procedures. Traditional industrial methods also use harsh reaction conditions in sulfuric acid, expensive platinum-based systems, increasing cost and reducing supply.
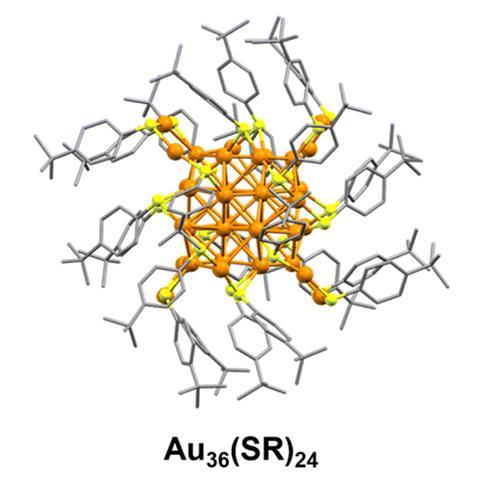
The catalyst is one of several gold–thiol clusters created by Yan Zhu, from Nanjing University, and her co-workers using a simple, inexpensive solution processing technique, with only crystallisation or chromatography required for purification.
In contrast to traditional methods, where the reaction involves adsorption and desorption of the reagent to proceed, this new process only involves a single adsorption step. All rearrangement steps then proceed with p-aminophenol desorbing from the catalyst cluster only at the end of the reaction.
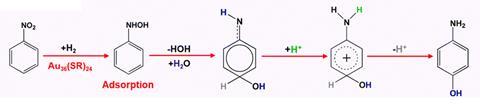
‘In their elegant work, the team has demonstrated that all reaction steps involved in nitrobenzene hydrogenation to p-aminophenol [actually] occur on the gold cluster surface,’ comments Cristina Della Pina from the University of Milan in Italy, who has recently developed a carbene-functionalised gold catalyst for reducing nitrobenzene. ‘Key is the presence of the benzenethiol (thiolate) ligands: when the ligands are removed from the gold cluster surface the selectivity was completely lost.’
Della Pina adds that the potential immediate impact of such a catalyst is apparent as gold catalysts are already used industrially. The fact that ‘the catalyst could be conveniently separated from the reaction system by centrifugation and reused with similarly high selective activity over seven consecutive cycles,’ offers a simplified approach with incredible robustness. Zhu’s team predicts ‘the studies of atomically precise metal cluster catalysts will lead to unexpected advances in catalysis and practical application.’
This work adds to other alternative syntheses for paracetamol being explored, including biological transformations and even recommissioning nerve agents, as manufacture is reshored globally to meet demand.
Potential applications of this technology do not stop with the pharmaceutical industry. Amines, particularly those with additional functionalities, are also essential in polyurethane manufacture, a $87 billion (£65 billion) market in 2023, as the use of toxic and environmentally hazardous isocyanates is phased-out.
References
This article is open access
J Lu et al, Chem. Sci., 2024, 15, 15617 (DOI: 10.1039/d4sc05018e)









No comments yet