Aluminium shows unprecedented selectivity for breaking toughest bond
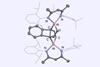
An aluminium complex reacts with tricyclic hydrocarbon’s most stable ring while ignoring easier-to-break carbon–carbon bonds
In a first for organometallic reactivity, aluminium has been found to selectively break biphenylene’s toughest carbon–carbon while leaving several weaker bonds intact.
Biphenylene consists of two benzene rings joined together by a pair of bonds, which creates a four-membered ring between them. This central ring is antiaromatic and, because it is so small, under a lot of geometric strain, making its bonds doubly weak and very reactive. In every bond-breaking reaction described so far, an organometallic reagent attacks the central ring and leaves the adjacent benzene rings intact.
