MOF-based material has detection limit as low as traditional Karl Fischer titration
Researchers from Germany have devised a new way to detect trace water in solvents. Their technique combines luminescent metal–organic frameworks (MOFs) with a magnetic core to give a system that users can see change colour then fish out of the sample once the test is complete.
Unwanted water is a big problem for some areas of chemistry. Many materials are water sensitive and must be kept in a dry atmosphere. And in some organic syntheses, water can initiate unwanted side-reactions, so it’s important that solvents contain as little water as possible.
‘There are two main molecules that can be addressed for sensing: water and oxygen. If you look at how many goods are transported worldwide, such as over the ocean, humidity sensing becomes very important,’ comments Klaus Müller-Buschbaum of the Julius Maximilian University of Würzburg, who led the work.
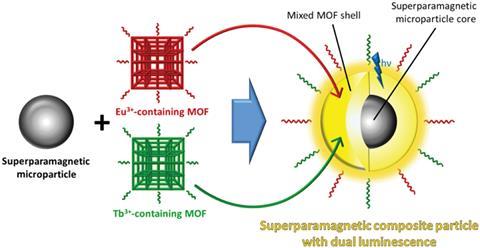
Müller-Buschbaum and his colleagues have made a material that combines two different MOFs, one containing terbium and one containing europium, with superparamagnetic Fe3O4/SiO2 microparticles. Water quenches the luminescent Tb3+ and Eu3+ centres unequally and concentrating the composite with a magnetic field means users can add it to test solvents to quantitatively determine how much water they contain by measuring then analysing the ratios of the luminescence bands.
‘We have the benefit that the magnetic properties allow us to have an additional chance to remove the sensor. We think that this is one of the exceptional things that this composite can do,’ says Müller-Buschbaum. ‘You can first disperse them and then concentrate them via a magnetic field. This is very good for strong signal augmentation,’ he adds.
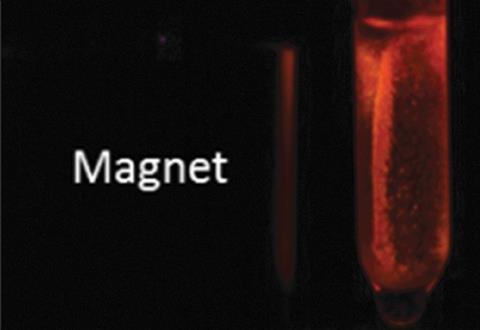
The composite is very sensitive, with a detection limit of 40μg/l. This is equal to the classic Karl Fischer titration method, which uses either colourimetric or volumetric titration to spot trace amounts of water in a sample. However, due to the equipment it requires, the Karl Fischer method is unsuitable for use outside of a laboratory. Müller-Buschbaum and his colleagues, however, are keen to emphasise that their new composites allow for ‘on-the-fly’ analysis.
Sujit Ghosh, who researches MOFs at the Indian Institute for Science Education and Research in Pune, India, is cautiously optimistic about the composites: ‘The crucial steps of sensitivity retention and cost-effectiveness are important hurdles to cross before such materials can compete with existing techniques. However, this work has the potential to overtake Fischer titrations.’
Raffaele Riccò, who researches MOFs and magnetic nanoparticles at Graz University of Technology in Austria, says that the composites have great potential: ‘Applications ranging from analytical methods to biomedicine can be foreseen.’ Ricco’s colleague, Paolo Falcaro, is also encouraged by the work: ‘Petrochemical and food industries could benefit from this exciting composite.’
References
This article is free to access until 20 November 2017
T Wehener et al, J. Mater. Chem. C, 2017, DOI: 10.1039/c7tc03312e









No comments yet