Cascade reaction brings a new dimension to amine synthesis
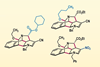
Method creates two new rings and four new bonds
A synthetic methodology developed by chemists in India can obtain complex caged amines from readily attainable azolium bromide salts through a cascade of annulation reactions. This method simplifies access to bicyclic scaffolds, previously hindered by tough syntheses, that could prove useful to drug developers.
