Copper catalyst behind general strategy for synthesising methylenecyclobutane compounds
Simple method that produces strained and synthetically-demanding molecules could be a boon for medicinal chemists
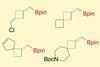
Researchers in China have developed a method for synthesising a variety of methylenecyclobutane derivatives. It overcomes previous synthetic challenges surrounding regioselectivity, stereoselectivity and functional group tolerance, so could make these underexplored compounds more accessible to medicinal chemists.
