Diels-Alder reaction directly observed under the microscope
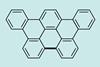
Simple ring-forming reaction followed on a surface for the first time using scanning probe microscopy
Every chemist knows the Diels-Alder reaction which produces a new ring of six carbon atoms without adding or removing any atoms, just by juggling six electrons. Now, scientists have achieved the difficult feat of carrying out the reaction on a surface and imaging each stage as it happens.1
The elementary version of the Diels-Alder, with a conjugated diene and an alkene or alkyne carrying few if any additional molecular groups, is one of the most studied in history. However, it has never been carried out on a surface. Therefore, nobody has been able to observe it directly using scanning probe microscopies.
