Silica nanoparticles with ultrahigh densities of silanol groups can bind many common medicines and release them in water, researchers in China have shown. The work could provide a widely applicable, cheap and non-toxic way to improve drugs’ solubility.

Solubility is a significant challenge for medicines, especially those delivered orally, and it is growing more acute with the development of new therapeutics. ‘Some of it’s a product of computational chemistry,’ explains formulation scientist Abina Crean at University College Cork in Ireland. This allows scientists to design a molecule to target a specific receptor, but she notes that ‘more lipophilic molecules generally interact better with target receptors’, and a drug is only effective if it is sufficiently soluble to get to the receptor it targets. Many solutions have been proposed, such as dispersion in solids, but these are often specific to one drug and can be unstable. No safe, low-cost and widely-applicable solution presently exists.
Silica is generally recognised as safe by the US Food and Drug Administration and naturally contains some silanol groups on its surface. These adsorb water so strongly that silica is used as a desiccant. They also have a natural affinity for carboxyl, carbonyl, amide and numerous other organic functional groups often present in drugs. This is sometimes added to tablets, but its ability to effectively bind and release drug molecules is limited because the density of silanol groups on the surface of silica is generally too low to extract drug molecules from the bulk crystals.
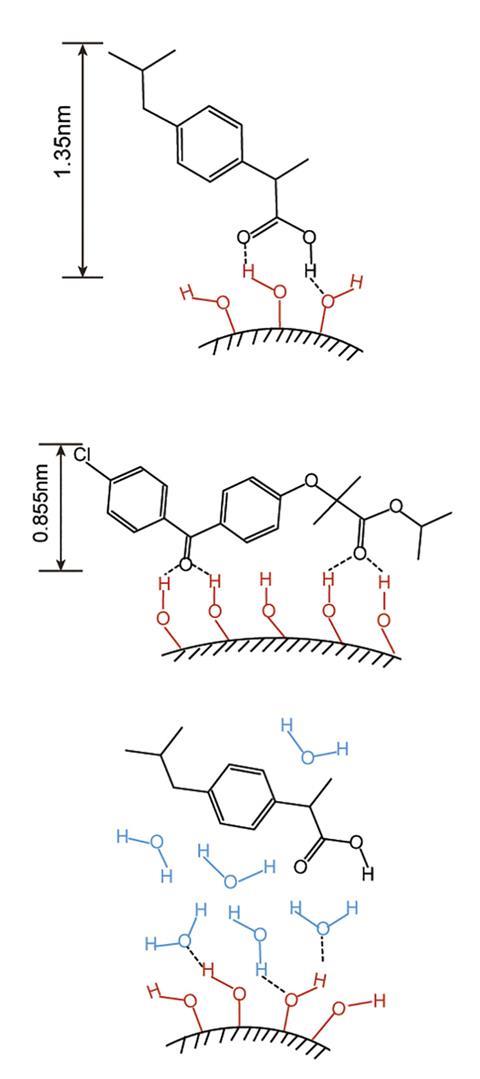
In the new work, the researchers created porous silica nanoparticles with ultrahigh silanol densities by evaporating aqueous colloidal suspensions under controlled temperature and pressure conditions. ‘We need this very high-curvature nanosurface because otherwise the silanol groups would interact with each other if they were too dense,’ explains physicist Lei Xu at the Chinese University of Hong Kong, who led the research together with David Weitz at Harvard University in the US. Multiple, tightly-packed silanol groups can form numerous hydrogen bonds to the same drug molecule. Adsorption to drugs was weaker than it was to water, however, so when the nanoparticles were added to water, the drug molecules were displaced and released into solution. The researchers tested this using drugs such as ibuprofen, simvastatin and diclofenac.
The researchers also tested the nanoparticles in mice, comparing ibuprofen delivered using their silica nanoparticles against the brand name ibuprofen medicine Nurofen. They found that their formulation achieved nearly double the peak plasma concentration and 1.5 times the overall exposure. ‘We think this could be a platform technology for many different drugs,’ says Lei. ‘Dave and I, together with our students who did most of the work, have formed a startup called PharmaEase Tech.’ He says they have received interest from industry.
Crean says that ‘there is good work here and the fundamental physics of what’s going on I don’t think has been presented this way before’. She is particularly impressed by the researchers’ ability to create and maintain long-lasting supersaturated solutions of the drugs. She notes, however, that ‘there is a lot of work to be done on whether this effect that they see in a pure aqueous system will translate into [gastro-intestinal] fluids, where you’d have more bile salts and different pHs’.
References
Z Xu et al, Proc. Natl. Acad. Sci. USA, 2025, 122, e2423426122 (DOI: 10.1073/pnas.2423426122)













No comments yet