Four-leaf clover molecule with metal centre is first in a new family of aromatics
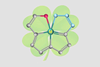
First molecule made of four fused aromatic rings around an osmium atom
A molecule resembling a four-leaf clover – four fused rings connected by a central osmium atom – is the first member of a new class of aromatics. The molecule has been constructed using reactions that had remained unexplored for more than two decades.
