Hydrogen bonded system faces strength test
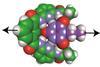
New tool can measure noncovalent interaction under non-equilibrium, near-physiological conditions
Scientists in Spain have devised a versatile technique that uses DNA to pull apart host–guest complexes so they can measure the overall strength of hydrogen bonds in that system. The method can distinguish forces as low as 0.1–1pN.