Observing orbitals solves hydrogen transfer puzzle
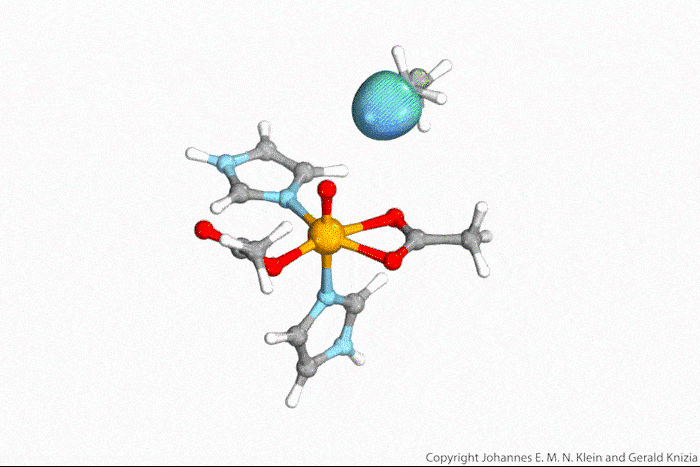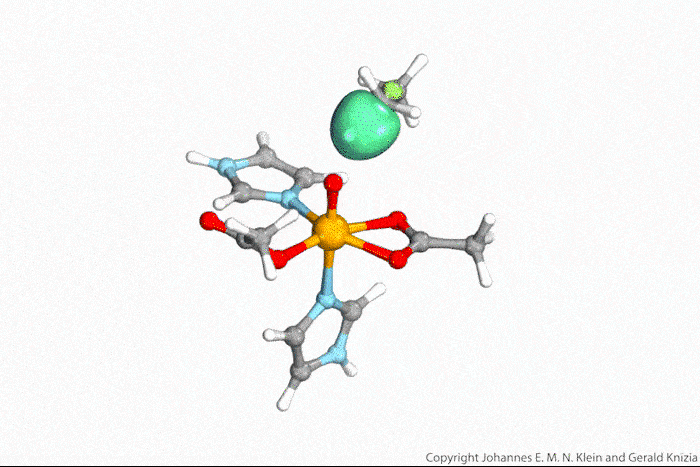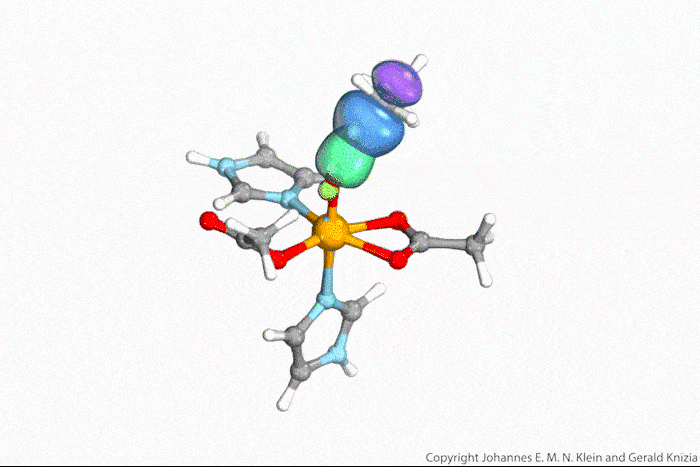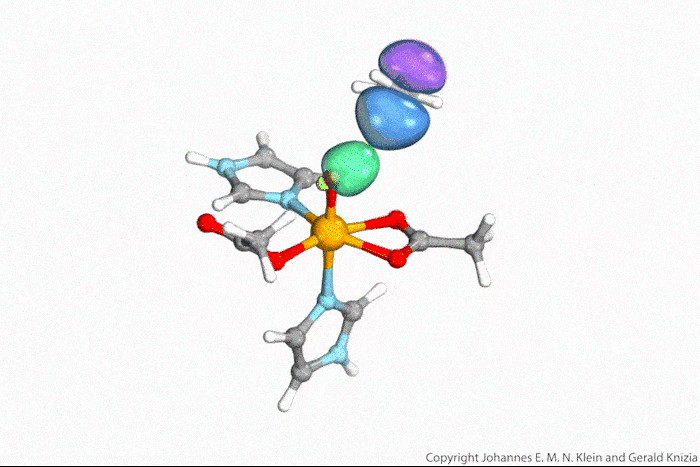
Visualising atoms’ wave function lets chemists tell true from indirect hydrogen atom transfer
Watching computed orbitals – visual representations of atoms’ quantum dynamics – dance around during a reaction might help chemists distinguish between different types of hydrogen atom transfers. This might save them from having to run kinetic experiments and build complicated models based on the results.
