Programmable peptoid template for disrupting protein–protein interactions inside cells
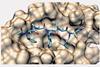
System optimises residues involved in binding target independently from those that promote membrane permeability
Scientists at the University of Tokyo in Japan have developed a peptoid-based system that can serve as a programmable template for making molecules that inhibit protein–protein interactions (PPIs). By overcoming some of the challenges in developing PPI modulators, the system could make it easier for researchers to study these underexplored drug targets.