Super-electrophilic ions enable selective modification of bioactive molecules
New technique will help medicinal chemists explore chemical space
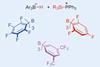
Researchers in the US have developed a new method for making site selective modifications to natural product molecules. The technique makes use of super-electrophilic silylium ions, and facilitates a range of different chemical transformations.
