Picking and choosing which bond to target holds the promise of transforming organic molecules at will
In the final holy grail paper, Bruce Arndtsen, Andrew Morley, Thomas Peterson and lead author Robert Bergman described selective intermolecular C–H bond activation as chemistry’s ultimate prize.
Notoriously stable carbon–hydrogen bonds are commonplace in almost all organic compounds. Being able to home in on individual bonds and replace a hydrogen with a group of one’s choosing is no mean feat, but it would offer chemists huge power to modify organic molecules at will.
How are you going to pick out one tree and cut it down without some kind of directing group?
Christina White, University of Illinois, Urbana–Champaign
In the decades prior to the Accounts issue, many researchers had established that transition metal complexes could cleave ‘unactivated’ C–H bonds – those bonds not situated near a heteroatom or an electron-rich carbon–carbon double or triple bond. The big challenge was to do this selectively and efficiently.
‘One thing does stand out about our experiences in those days – it was all stoichiometric chemistry,’ recalls Bergman. ‘And I talked to lots of people who said, “I enjoyed your article, but what we really need is catalytic C–H activation, and this will never be catalytic. Nobody will ever be able to turn this into catalytic chemistry.” Which turned out, of course, to be completely wrong.’
The first evidence of catalytic C–H activation in alkanes was discovered in 1969 when Alexander Shilov’s lab at the Institute of Problems of Chemical Physics in Chernogolovka, Russia, showed that a simple platinate system could bring about hydrogen–deuterium exchange in methane and ethane.
In 1982, Bergman and his then postdoc Andrew Janowicz reported another major advance when, for the first time, they directly observed a C–H activation of a fully saturated hydrocarbon by a soluble organometallic system. Their discovery of an iridium complex capable of inserting itself into simple alkanes was quickly followed by a report of a similar system by William Graham’s group at the University of Alberta, Canada.
By the time Bergman was asked to write the holy grail article, several metals had been used in C–H activation. For him, the main aim now would be to create a highly active catalyst able to convert cheap and abundant fossil fuel products like methane into more versatile feedstocks like methanol.
Many researchers, for example the Scripps Institute’s Roy Periana (one of Bergman’s former graduate students), have discovered numerous new systems capable of driving these reactions. But 25 years later, we’re still searching for a catalyst with a high enough turnover to make C–H activation chemistry practical on an industrial scale. However, Bergman stresses that important advances have been made in understanding the catalytic cycles involved. ‘And one of the most important, in my view, is Graham Ball’s work where he’s been able to use NMR spectroscopy at very low temperatures to actually identify and observe alkane–metal complexes, and to make extremely specific decisions about what the structures of the complexes really are.’
New applications
Bergman notes that perhaps the most significant advances have come in the applications of these reactions. Something that wasn’t clear in 1995 was how C–H activation could be used on much more complex compounds. If synthetic chemists could selectively activate C–H bonds on highly functionalised molecules, it would enable them to create and investigate much more valuable products such as agrochemicals or drugs. But how could you target a single site in a large molecule containing dozens of carbon–hydrogen bonds? For many this seemed an almost impossible goal.
‘This was very ingrained in the mentality of researchers at the time, they sort of viewed it as though C–H bonds are like a forest of trees – how are you going to pick out one tree and cut it down without some kind of directing group or molecular recognition element as shown in the cases that worked in nature?’ says University of Illinois at Urbana–Champaign’s Christina White.
Several synthetic chemists had tried to copy motifs seen in enzymes like methane monooxygenase and iron–haem P450 systems that carry out highly selective alkane functionalisations. But White explains that initial attempts to mimic these enzymes’ structures didn’t recreate their activity. ‘Really, my guiding principle was that we wanted to mimic the behaviour of these biological systems, not necessarily the way they looked,’ she says.
When White established her lab in 2002, her team began testing non-haem iron complexes for C–H activation. In the course of these investigations, White and her then graduate student Mark Chen took a tetradentate ligand and made a series of modifications to increase its rigidity. They found that this significantly improved the catalyst’s activity and selectivity, allowing precise oxidation of unactivated C–H bonds on saturated hydrocarbon chains on a scale that would be useful for synthetic chemists.
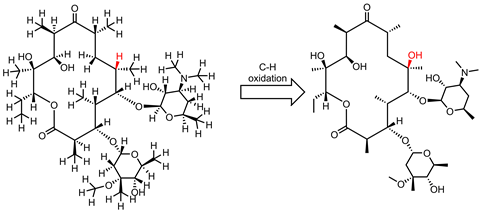
In 2007, White and Chen published their results in Science showing that their iron-PDP system, containing one large tetradentate unit and two acetonitrile ligands, could use subtle differences in sterics and electronics to select for different C–H bonds in an array of complex organic compounds. Remarkably the catalyst was able to carry out a selective C–H oxidation on the antimalarial drug artemisinin faster than a P450 enzyme.
In the years that followed White’s lab continued to develop variants of the PDP system, producing a number of iron and manganese catalysts that promote a wide range of C–H oxidations on complex substrates, including peptides, alkaloids and amides.
Within touching distance
‘Now, for the first time, we have one of the goals that Bergman set out, which was to understand – he calls it the “energy surfaces” for C–H activation, especially for relatively complex molecules,’ says White. ‘And I think now we do, right? We know that the C–H bonds are not all the same, even though their bond dissociation energies may tell a different story.’
Other researchers have focused on catalysts that make use of common features of organic molecules to direct site-selective reactions. Jin-Quan Yu’s group at the Scripps Research Institute in California has focused on palladium-based catalysts that allow enantioselective C–H functionalisations. ‘Our vision in 2002 was to harness weak coordination of substrate to metal for directing C–H activation – that has served as the one of the cornerstones for our research,’ says Yu. By opting for this weak interaction between metal and substrate, Yu hoped to ensure that any reactivity would be driven by the ligand. This would limit unwanted side-reactions and maximise the role that the ligand’s structure could play in imparting chirality.
Yu’s group published initial results of their monoprotected α-amino acid ligands in 2008, showing that these systems could promote highly enantioselective C–H alkylation reactions. Since then Yu’s lab has developed many new generations of ligands that have hugely expanded the potential of C–H activation chemistry.
‘It’s not just substrate scope, but also it has broadened the reaction type. We’re not doing just one reaction – we can do cross coupling, we can do hydroxylation chemistry,’ says Yu. ‘Now we’re the first group that can couple sp3 C–H bonds like [a Heck reaction] with double bonds.’
The success of these systems has seen all of White and Yu’s ligands extensively commercialised by pharma companies who use the technology to perform late-stage functionalisation on lead molecules. This enables them to create many different analogues of potential drug candidates to quickly analyse the impact of different functional groups on a compound’s bioactivity.
These discoveries, and others from researchers like Melanie Sanford, Frank Glorius, John Hartwig and Lutz Ackerman, have contributed to a blossoming of the field of C–H activation. In 1995 fewer than 100 papers on C–H activation were published. In 2019 there were more than 12 times that number.
So what does the future hold?
For White the goal is to make C–H oxidation as useful in drug discovery as cross coupling reactions are today. However, she notes that there are challenges involving chemoselectivity with certain substrates and in controlling reaction rates.
Yu points out that selective C–H activation is still relatively new compared with double bond chemistry, and believes that many more ligand classes need to be synthesised to fully realise its potential. ‘In the last five years there’s a sharp increase of applications in both synthesis and medicinal chemistry, but I still think it should be faster. And the reason is because not many people are developing new ligands to accelerate [the field],’ he says.
Bergman still hopes that a highly active catalyst class can be developed to functionalise simple alkanes on massive scale. He thinks that a better understanding of the common chemistry in homogeneous and heterogeneous systems would be a significant step towards this.
‘It’s always kind of perplexed me that the way that people study heterogeneous reactions, many chemical engineers for example, they do it using a completely different language than organometallic chemists use,’ he says. ‘So that’s one of the things that’s developed a barrier between the two fields. There must be similar principles that govern what controls heterogeneous and homogeneous reactions, but the principles that people are uncovering in both fields don’t mesh very well.’
‘The way the kinetics are done is different, the kinds of techniques that are used to study these systems are different,’ Bergman adds. ‘So I would say that if that can be solved – I don’t want to prognosticate that it will be solved – that would be a major achievement, at least in understanding these processes.’
Update 2 October 2020: The cumulative citation charts and their captions have been updated to include papers that had been omitted from our original dataset, and Bruce Arndtsen’s name has been corrected.
Cumulative cites caption: Due to the huge scope of C–H activation chemistry and the many different types of catalysts that have been investigated in this class of reactions, all of the top 20 papers in terms of citations are review articles. In fact, only three articles in the top 50 are not reviews. In top place, with a massive 4505 citations, is a 2010 article by Thomas Lyons and Melanie Sanford describing palladium-catalysed ligand-directed C–H activations.
Cumulative cites caption: Due to the huge scope of C–H activation chemistry, our initial search found the top 20 papers in the field was made up almost entirely of review articles. Here, we have excluded reviews from our search, so that you can see the most cited research communications. Strikingly, all of these papers were published after 2000, reflecting the explosion of the field once it became apparent that this chemistry could be used in late-stage functionalisation of complex molecules. Christina White’s 2007 paper on the iron-PDP system appears in fifth place with 831 citations. It is interesting to note that four of the top 20 papers were published by the group of Keith Fagnou, a Canadian organic chemist who passed away at an early age in 2009.
Citation network caption: Here we can see researchers connected to other chemists who have cited their work. The larger a researcher’s circle, the more times they have been cited in key papers on C–H activation. The network appears to show a very large and interconnected field in which researchers regularly cite each other’s work. The most commonly cited author is Jin-Quan Yu, who appears just below the centre of the main cluster. Other highly cited researchers include Melanie Sanford, Masahiro Miura and Robert Bergman, among others.
Co-author network caption: This chart shows the authors of highly cited C–H activation papers connected to their co-authors. The colours denote when each researcher’s earliest highly cited paper was published across the period 1995–2020. Point size reflects the number of key papers each author has published. By moving through the five-year groupings, it is clear to see the explosion of interest in the topic from the late-2000s as researchers around the world seek to use this chemistry in the functionalisation of complex molecules.
Geo caption: In this chart we can see the locations of researchers publishing high-impact papers on C–H activation. Early interest in the topic appears centred in Europe, the US and Japan, while we can see a surge in interest around the globe as key papers are published from the late-2000s onwards.
Additional information
For an explanation of how these data visualisations were built, read Behind the data
Further reading
1 M Chen et al, Chromium(iii)-catalyzed C(sp2)–H alkynylation, allylation, and naphthalenation of secondary amides with trimethylaluminum as base, J. Am. Chem. Soc.., 2020, 142, 4883 (DOI: 10.1021/jacs.0c00127)
2 H Huang, Z M Strater and T H Lambert, Electrophotocatalytic C–H functionalization of ethers with high regioselectivity, J. Am. Chem. Soc., 2020, 142, 1698 (DOI: 10.1021/jacs.9b11472)
3 X Leng et al, Direct observation of meta-selective C–H activation on Pd(111) by scanning tunneling microscopy, Chem. Phys., 2020, (DOI: 10.1016/j.chemphys.2020.110981)
4 E Casali et al, Overriding ortho selectivity by template assisted meta -C–H activation of benzophenones,Chem. Commun., 2020, 56, 7281 (DOI: 10.1039/d0cc03172k)
5 R Oeschger et al, Diverse functionalization of strong alkyl C–H bonds by undirected borylation, Science, 2020, 368, 736 (DOI: 10.1126/science.aba6146)
6 Z Fan et al, Merging C(sp3)–H activation with DNA-encoding, Chem. Sci., 2020, DOI: 10.1039/d0sc03935g
7 M E Potter et al, Cobalt-containing zeolitic imidazole frameworks for C–H activation using visible-light redox photocatalysis, Catal. Sci. Technol., 2020, DOI: 10.1039/d0cy01061h
8 J Zhang, C Tang and Z Xie, Magnesium-mediated sp3 C–H activation in cascade cyclization of 1-arylethynyl-2-alkyl-o-carboranes: efficient synthesis of carborane-fused cyclopentanes, Chem. Sci., 2020, DOI: 10.1039/d0sc04465b
9 Y Li et al, Palladium(ii)-catalyzed asymmetric C–H carbonylation to diverse isoquinoline derivatives bearing all-carbon quaternary stereocenters, Chem. Commun., 2020, DOI: 10.1039/d0c05219a
10 K Niu et al, C–H activation of light alkanes on MXenes predicted by hydrogen affinity, Phys. Chem. Chem. Phys., 2020, 22, 18622 (DOI: 10.1039/d0cp02471f)
Searching for the holy grails of chemistry

How has science progressed over 25 years?
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
 Currently
reading
Currently
reading
Seeking out selective C–H activation


























No comments yet