Challenging mirror molecules made with stereochemistry destroying reaction
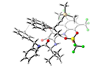
Clever catalyst repurposes classic nucleophilic substitution to make chiral quaternary carbon centres
A substitution reaction known for destroying stereochemistry has been converted into a chirality-creating reaction to tackle one of the toughest organic chemistry challenges: making quaternary carbon centres.
There is currently no perfect way to make quaternary stereocentres. Most methods rely on using a pro-chiral substrate – a compound like a tri-substituted alkene with well-defined stereochemistry. But making these pro-chiral substrates is a challenge in itself.
