The nature of an ‘unidentified product’ in drinking water disinfected with chloramines, which serves over 113 million people in the US alone, has finally been revealed by researchers in the US and Switzerland. First detected almost 40 years ago, the molecular ion, called chloronitramide, has never been characterised before, so its toxicology is unknown. However, its concentration in some samples of tap water taken in areas such as California and Texas and its similarity to harmful molecules warrant investigation, the researchers warn. The discovery could also add to previous concerns about the use of chloramines to sterilise tap water.
Chlorine, a low-cost and highly effective disinfectant, is the most widely used chemical for steralising drinking water across the world. But in the 1970s, scientists found that it could react with organic matter to produce harmful chemicals such as trihalomethanes and haloacetic acids. The US Environmental Protention Agency (EPA) subsequently regulated the maximum permissible concentrations of several of these in tap water. To reduce the production of these disinfection by-products, many water treatment authorities in the US and some other countries including the UK have partially or totally switched to disinfecting water with chloramines, which are formed by combining chlorine with ammonia.
In the early 1980s the signal of a mysterious molecule was observed in UV absorbance spectra of chloraminated water. Environmental engineer Richard Valentine at the University of Iowa, US, and his colleagues conducted extensive investigations in the 1980s and 1990s, showing the circumstances under which it formed. However, the molecule’s exact structure remained unclear.
‘We showed that there was a major product formed when monochloramine decayed – which it does naturally, and it had to be in quite high concentration,’ explains Valentine. ‘What made it difficult is that it had the same ultraviolet spectra as the reactant monochloramine.’ He compares looking for the structure as ‘like looking for a needle in a haystack where all the pieces of hay are needles’.
In the new research, environmental engineers led by Julian Fairey at the University of Arkansas, US, collaborated with analytical chemists at ETH Zurich led by Juliana Laszakovits. The researchers used multiple technologies to which Valentine’s group had not had access, such as ion chromatography coupled to electrospray ionisation–mass spectrometry. ‘This combination of techniques isn’t so commonly used in environmental studies, but it’s really good at separating anions,’ says Laszakovits.
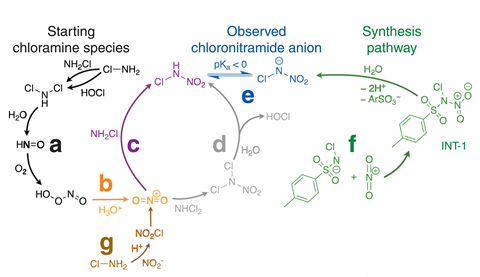
The molecule was too small for its structure to be determined by its mass spectrometry fragmentation pattern. But mass spectra of isotopically labelled samples allowed the researchers to confirm that it contained two oxygen atoms, two nitrogen atoms and a chlorine atom. 15 N NMR and infrared spectroscopy then helped to confirm its structure.
The researchers’ experiments suggest that the compound principally forms in chloraminated drinking water by hydrolysis of dichloramine to nitroxyl, which reacts with dissolved oxygen to produce peroxynitrite. ‘One of the decomposition products of peroxynitrite is a nitrating agent called the nitronium cation, and that’s the linchpin that then reacts with either monochloramine or dichloramine to produce this compound,’ says Fairey.
As the molecule has never been isolated before, its toxicity is unknown. However, the researchers used an EPA algorithm called Generalized Read Across to compare its structural and chemical similarities to known molecules. They concluded further study is urgently warranted, especially given that concentrations in some of their samples are higher than those of regulated hazardous compounds. In fact, Fairey originally began the project studying how nitrosamines – a class of compounds that are considered ‘probable carcinogens’ – form in chloraminated water. ‘If you removed one of the oxygen atoms from the nitro group, you’d have a nitrosamine,’ says Kristopher McNeill at ETH Zurich in Switzerland – where chloramination of water is illegal.
‘I think it’s excellent work by the exact people to figure it out’, says Valentine. ‘I’ve been waiting 40 years for someone to pick up on my work.’
Environmental engineer Daniel McCurry of the University of Southern California, US, says that the finding is ‘very significant’ and believes that it should prompt a re-evaluation of the use of chloramine as a water disinfectant. He notes other disadvantages such as a greater propensity to corrode lead pipes and unpleasant ‘pool smell’. ‘Any plant in the US could sufficiently treat their water with chlorine without violating disinfectant byproduct rules if they were willing to and had the resources to add certain other treatment steps like activated carbon or membranes.’ he says. ‘They’re probably going to have to do that anyway because of PFAS regulations. So hopefully this might be a two birds, one stone situation.’
Correction: This article was updated on 25 November 2024. An earlier version incorrectly stated that the chloronitramide anion contained only one nitrogen atom.
References
J L Fairey et al, Science, 2024, DOI: 10.1126/science.adk6749

















No comments yet