Flow synthesis produces chiral intermediate for antidepressant drug
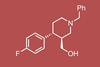
The first solvent-free organocatalytic flow process yields the key chiral intermediate for paroxetine on a multigram-scale
Researchers in Austria and Spain have collaborated to develop a solvent-free continuous flow synthesis for a key chiral intermediate of the antidepressant drug (–)-paroxetine. The process employs robust catalysts and intensified flow conditions to generate this compound on a multigram per hour scale.